* Generally impractical with fluoxetine due to the protracted interval needed for washout of that SSRI and its effect on CYP enzymes.
| Preskorn.com | Printed from: http://www.preskorn.com/books/ssri_s8.html |
| Clinical Pharmacology of SSRI's 8 - How Does This Knowledge Relate to the Clinical Use of SSRIs? |
|
|
|
|
The effect of specific serotonin selective reuptake inhibitors (SSRIs) on specific cytochrome P450 (CYP) enzymes has been the major distinguishing factor among these drugs and is clinically important for three reasons:
For all of these reasons, there is a substantial likelihood that the patient who is taking an SSRI will be treated with another medication and hence will be at potential risk for a drug-drug interaction.
Consistent with these observations, several recent surveys have shown that many patients being treated with an antidepressant were also taking other medications and, hence, had the potential to experience a drug-drug interaction (Table 8.2).
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
As discussed in Section 3, one of the goals in the rational development of the SSRIs was to reduce the likelihood of pharmacodynamic drug interactions. Originally, the goal was to avoid the multiple types of pharmacodynamic drug interactions that can occur when using tricyclic antidepressants (TCAs) in combination with other drugs due to the multiple mechanisms of action (MOAs) of TCAs. While SSRIs were developed to avoid this problem, they were developed before we had the ability to screen for effects on the CYP enzymes. This fact explains why there are so many differences among SSRIs with regard to their effects on these enzymes. In essence, the action of some of the SSRIs on these enzymes is analogous to the effect of TCAs on fast sodium channels: these effects are unintended and unnecessary relative to the desired goal of treating major depression. Instead of adding to their efficacy, the inhibition of these enzymes produces the undesired risk of causing pharmacokinetic drug interactions. This section will focus on the differential effects of SSRIs on specific CYP enzymes and their potential for causing such interactions.
A separate issue is whether the inhibition of these enzymes has any long-term consequences (Table 8.3). This issue, while only theoretical now, is potentially important since patients may remain on SSRIs for years to prevent recurrent depressive episodes. For example, prophylactic treatment with fluoxetine and paroxetine essentially converts the patient into a phenocopy of genetic deficiency of the CYP enzyme 2D6 (Table 8.4). Due to the widespread use of SSRIs for maintenance treatment, a relatively large segment of the population is being converted into phenocopies of CYP 2D6 deficiency. Obviously, this enzyme is not essential to life given the rather widespread (approximately 7%) incidence of its deficiency in Caucasians. However, it will be important to know whether functional activity of this enzyme is a risk factor for the development of chronic illnesses.3,53,55,61,88,138,231 This issue requires specific study as discussed in Section 7.
Myths Concerning SSRIs and CYP Enzymes
Perhaps as a result of the intense competition among these drugs, there has been a considerable amount of disinformation about the issue of CYP enzymes and SSRIs. A number of myths have arisen that may cause confusion (Table 8.5). Many of these myths involve the effects of these drugs on the enzyme CYP 2D6, probably because this enzyme was the first one shown to be substantially inhibited by some SSRIs. One myth is that the inhibition of these enzymes, particularly CYP 2D6, is a class issue with SSRIs. A subset of this myth is that only the in vitro potency of these drugs needs to be compared to determine their relative clinical effects without regard to the different concentration of different SSRIs achieved under clinically relevant conditions. A second myth is that the inhibition of these enzymes affects only one or perhaps a couple of drugs. A third myth is that all the drugs affected by the inhibition of a specific CYP enzyme are known.
| TABLE 8.4 — Some SSRIs Lack Selectivity With Regard to Effects on Serotonin Uptake Versus CYP 2D6 | ||||
| SSRI | Usually Effective, Antidepressant Dose (mg/day)1 | Plasma Level (ng/ml)1 | Serotonin Uptake1 | CYP 2D62 |
| Citalopram | 40 | » 85 | » 60% | » 15% |
| Fluoxetine | 20 | » 200 | » 80% | » 85% |
| Fluvoxamine | 150 | » 100 | »70% | < 15% |
| Paroxetine | 20 | » 40 | » 80% | » 85% |
| Sertraline | 50 | » 25 | » 80% | » 15% |
| 1See Table
3.7. 2See Table 8.9. |
||||
In this section, we will address these myths by reviewing our current knowledge of the effects of the SSRIs on specific CYP enzymes. We will first review how this information has been developed so that the reader can understand, and even anticipate, future developments in our knowledge about the relative effects of drugs, SSRIs and others, on these enzymes. While this book is on SSRIs, the issue of drugs affecting CYP enzymes is not restricted to this class. They are merely examples of this phenomenon and thus serve to illustrate a larger point.
|
||
|
How Do We Determine the Effects of Specific Drugs on Specific CYP Enzymes?
Our knowledge in this area comes from both in vitro and in vivo studies (Table 8.6). In vitro studies can be used to determine how potent a specific drug is as an inhibitor of a specific CYP enzyme. In vivo studies are done to determine whether the interaction occurs and to what extent under clinically relevant conditions. A brief description of the two different approaches follows as a frame of reference for the remainder of this section.
In Vitro Studies
Several different techniques can be used in these studies, including the use of:
These techniques can be used to determine whether a specific human CYP enzyme is responsible for the metabolism of specific drugs and whether specific drugs are capable of altering the functional activity of a specific human CYP enzyme. Presently, the most common approach employs microsome preparations. After an appropriate series of samples have been prepared, a substrate (eg, a drug known to be metabolized by that enzyme) for the enzyme is added in a predetermined concentration. Then, the potential inhibitor is added in varying concentrations to determine its potency for blocking the enzyme-mediated biotransformation of the substrate. This approach is analogous to determining the binding affinity of a drug to a specific neuroreceptor (see Section 3). The results of such in vitro studies for the various SSRIs for 3 important CYP enzymes are shown in Table 8.7.
While the in vitro potency is an important determinant of the potential for a drug to produce an effect, it is not the sole determinant. Obviously, the drug must reach a sufficient concentration at the site of action (SOA) (which is the CYP enzyme in this instance) to produce sufficient inhibition of the enzyme to be clinically meaningful. In the case of a pharmacokinetic interaction, this means the inhibition of the enzyme must be sufficient to cause a clinically meaningful change (either an increase or decrease depending on the interaction in question) in the plasma and tissue concentration of a concomitantly administered drug which is dependent on that CYP enzyme for its clearance. There is no simple way to determine whether the degree of inhibition that will be achieved by two drugs will be the same or different based solely on their relative in vitro potency for inhibiting an enzyme. Instead, the relative concentrations of the drugs under clinically relevant conditions must be taken into account. Moreover, it is not the plasma drug concentration that is critical but rather the concentration at the enzyme.
Mathematical modeling can be done to estimate the degree of enzyme inhibition that will be produced by a given drug under clinically relevant conditions based on a knowledge of 3 factors:
Many readers may not be interested in more details concerning such modeling work beyond knowing that it exists and what its implications are. For those who are interested in more details, see Section 10, Appendix.
The important application of such modeling is that it can help to determine whether a specific pharmacokinetic interaction is likely to occur to a clinically meaningful extent when that drug is coprescribed with a drug that is dependent on that enzyme for its elimination. This approach can help to rationally guide research to focus on the most critical pharmacokinetic studies (ie, those which are likely to reveal an important interaction). This is a significant advance over the previous approach where drugs were studied simply because they had a higher likelihood of being coprescribed together or because interactions had been described with other drugs in a "class" such as "antidepressants" which includes vastly different drugs such as TCAs, SSRIs and monoamine oxidase inhibitors (MAOIs).
Although such modeling work is an important screening tool to focus research on highly likely interactions, a definitive answer as to whether a drug will inhibit a CYP enzyme to a sufficient extent to produce a clinically meaningful drug-drug interaction requires formal in vivo pharmacokinetic interaction studies done under clinically relevant dosing conditions. In such studies, the degree of enzyme inhibition is typically measured by changes in the pharmacokinetics of a drug dependent on a specific CYP enzyme for its clearance in the absence, and then in the presence, of the potential inhibitor.
| TABLE 8.7 — The Relative Potency* of Five Different SSRIs and Their Metabolites for Inhibiting the Functional Integrity of Three CYP Enzymes 1A2, 2D6 and 3A3/4 Based on In vitro Studies Using Human Hepatic Microsomes | |||||
| Study | Citalopram / desmethyl-citalopram | Fluoxetine / Norfluoxetine | Fluovoxamine | Paroxetine / M2 | Sertraline / desmethyl-sertraline |
| CYP 1A2 | |||||
| Brosen et al1 | >100/ >100 | >100/ >100 | 0.2 | 45/NA | 70/NA |
| Rasmussen et al2 | >100/ >100 | >100/ >100 | 0.2 | 50 | >100 |
| von Moltke et al3 | NA | 4.4/5.9 | 0.24 | 5.5 | 8.8/9.5 |
| CYP 2D6 | |||||
| Crewe et al4 | 5.1 | 0.60/0.45 | 8.2 | 0.15/0.50 | 0.70/NA |
| Skjelbo et al5 | 19/1.3 | 0.92/0.33 | 3.9 | 0.36/NA | -- |
| Von Moltke et al6† | -- | 3.0/3.5 | 16.6 | 2.0/NA | 22.7/16.9 |
| Otton et al7 | -- | 0.17/0.19 | -- | -- | 1.5/NA |
| Otton et al8 | -- | 0.15/NA | -- | 0.065/NA | 1.2/NA |
| CYP 3A3/4† | |||||
| Von Moltke et al9 | -- | 83.3/11.1 | 10 | 39/NA | 23.8/20.4 |
| Rasmussen et al2 | >100/ >100 | 60/19 | 40 | 70 | 90 |
| * In vitro
potency = Ki = inhibition constant; the smaller
the value, the greater the potency on a molar basis. † Ki values for comparison: quinidine = 626; ketoconazole = 0.0510 |
|||||
| NA = Not applicable | |||||
| References: 147, 2235, 3299, 464, 5256, 6278, 7196, 8194, 9279, 10276 | |||||
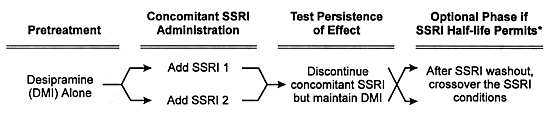
In Vivo Studies
The general design for a formal in vivo pharmacokinetic study is given in Figure 8.1. The essence of these studies is to determine whether the coadministration of the potential inhibitor or inducer alters the clearance of a concomitantly administered drug that is a model substrate for the enzyme in question. Ideally, such studies use a model substrate which is a drug that is solely metabolized by one CYP enzyme and does not itself alter this enzyme's activity (ie, no autoinduction or autoinhibition) over the clinically relevant concentration range.
The first step is to give the model substrate to normal volunteers to determine its rate of biotransformation and elimination (ie, its clearance) in the absence of the potential inhibitor or inducer. Two different dosing strategies have been used with regard to the administration of the model substrate.
In the single dose approach, the clearance of the model substrate in a volunteer is quantitated after a single dose in the absence and presence of the potential inhibitor or inducer. The latter is usually dosed to steady-state prior to the single-dose rechallenge of the model substrate. In the multiple dose approach, the model substrate is administered to the volunteer on a daily basis for a sufficiently long enough interval to ensure that its steady-state has been achieved. The potential inhibitor or inducer is then added to the regimen at a clinically relevant dose and administered sufficiently long enough to ensure it has also reached steady-state conditions. The clearance of the model substrate is measured before and after the addition of the potential inhibitor or inducer. The multiple dose approach is more rigorous and more expensive, and more closely mimics clinical practice than does the single dose approach.
In both approaches, the potential inhibitor or inducer is administered on a multiple dose basis in an analogous way to how it would be given in clinical practice. Changes in the plasma levels and clearance of the model substrate quantitatively reflect the degree of enzyme inhibition or induction produced by the potential inhibitor or inducer respectively under clinically relevant conditions.
| FIGURE 8.1 — Design of Pharmacokinetic Interaction Studies Using SSRIs as an Example |
 |
|
* Generally impractical with fluoxetine due to the protracted interval needed for washout of that SSRI and its effect on CYP enzymes. |
In the past, in vitro and in vivo studies have been done in a nonsystematic, nonsequential fashion. For example, in vivo studies might be done before in vitro studies or, even though an in vitro or in vivo study might be positive, the other type of study might not be done. An in vitro study might show that a specific drug has an inhibition potency such that one would expect that the effect could occur under clinically relevant conditions, but the confirmatory in vivo studies might not be done. For these reasons, there are gaps in our current knowledge, but they are in the process of being filled.
The trend now is for these studies to be done using a more sequential approach. For efficiency, in vitro studies are done first to screen for potentially meaningful effects of an investigational drug on a specific CYP enzyme. Those studies determine the in vitro potency of the investigational drug for inhibiting or inducing the enzyme. This information, coupled with a knowledge of what concentration of the investigational drug is achieved under clinically relevant conditions, can be used to predict whether this drug is likely to produce meaningful inhibition or induction of the enzyme under clinically relevant conditions. Then, in vivo studies are done to confirm cases where it is likely a pharmacokinetic drug interaction will occur under such conditions. The in vivo follow-up studies are done with drugs (ie, model substrates) that can serve as in vivo probes to quantify the functional activity of the enzyme before and after the coadministration of the investigational drug (ie, the potential inhibitor or inducer). The results of the in vivo studies can also be used to predict whether other drugs that are substrates for that specific CYP enzyme will be affected when they are coadministered with the investigational drug under clinically relevant conditions.
Obviously, this approach is not restricted to SSRIs but can be used with any drug suspected of affecting a specific CYP enzyme. Research with SSRIs has simply been pioneering work in this area and has documented that several SSRIs are capable of inhibiting one or more CYP enzyme to a clinically meaningful extent under clinically relevant conditions.
Beyond this systematic approach to detecting potential pharmacokinetic drug interactions, spontaneous observation in clinical practice can lead to case reports. These reports can serve the useful purpose of guiding formal research, particularly when there is in vitro data that also supports the probability of an interaction under clinically relevant conditions. Nonetheless, case reports are only suggestive and not definitive and clearly cannot be equated with the results from formal pharmacokinetic studies due to the limited amount of data and the typical absence of adequate controls. For example, there is often no control for compliance in such case reports and the interpretation is highly dependent on the validity of the first sample obtained, which is typically done on an outpatient basis.
What Do We Know About the Effects of Specific SSRIs on Specific CYP Enzymes?
The results from the studies that have been done on 5 major xenobiotic CYP enzymes (ie, CYP 1A2, 2C9/10, 2C19, 2D6 and 3A3/4) follows and is also summarized in 2 tables. Table 8.8 presents a composite summary based on the available formal in vitro and in vivo studies. As can be seen from the table, there is a variable amount of data with regard to both a specific enzyme and the effects of a specific SSRI. Data relative to a specific CYP enzyme may be available for some, but not all, SSRIs. Data may come solely from either in vitro or in vivo studies and sometimes data from both types of studies are available, which is the ideal situation. Occasionally, only suggestive case reports are available, either alone or combined with in vitro studies. Again, data based on case reports, particularly when limited to a small number of patients, must be interpreted in a cautious manner and not equated with results from formal, rigorous studies. Table 8.7 summarizes the results of the in vitro studies that have been done with the SSRIs relative to three specific enzymes: CYP 1A2, 2D6 and 3A3/4. Tables 8.9 and 8.11 summarize the in vivo studies.
| TABLE 8.8 — Effects of Specific SSRIs on Specific CYP Enzymes at Their Respective, Usually Effective, Antidepressant Dose* | ||||||
| Enzyme | Citalopram | Fluoxetine | Fluvoxamine | Paroxetine | Sertraline | |
| CYP 1A2 | Unlikely1 | Unlikely1 | Substantial1,2 | Unlikely1 | Unlikely1 | |
| CYP 2C9/10 | ? | ?3 | ? | NCS2 | NCS2 | |
| CYP 2C19 | ? | Moderate2 | Substantial2 | ? | NCS2 | |
| CYP 2D6 | Mild1 | Substantial1,2 | NCS1,2 | Substantial1,2 | Mild1,2 | |
| CYP 3A3/4 | ? | Mild1,2 | Moderate1,2 | Unlikely1,2 | Unlikely1,2,4 | |
| ? = absence of or
contradictory in vitro or in vivo data available for this
SSRI Unlikely = based on in vitro studies, unlikely to have a clinically meaningful effect NCS = not clinically significant in most situations = < 20% change†
Moderate = 50% to 150% change† Substantial = > 150% change† |
||||||
|
* Table is a summary of the results presented in Tables 8.7, 8.9, 8.11, and 10.1, and data reviewed in the text. Hence, it is based on effects observed or predicted based on the concentration of these drugs that would be usually produced by their usually effective, antidepressant dose. Since the inhibition of these enzymes is concentration-dependent, the magnitude of the effect will be higher on average at higher doses particularly for SSRIs with nonlinear pharmacokinetics (see Table 6.2). |
||||||
|
||||||
The data regarding the differential effects of SSRIs on CYP 1A2 come principally from 2 in vitro studies (Table 8.7). Brosen et al and Rasmussen et al have shown that fluvoxamine has an inhibitory rate constant for this enzyme such that it is likely to cause clinically meaningful inhibition under antidepressant treatment conditions (Table 8.7). Consistent with these in vitro findings, there is evidence that fluvoxamine inhibits the clearance of drugs dependent on this enzyme for biotransformation prior to elimination. While the rate-limiting step for the elimination of all TCAs is ring hydroxylation mediated by CYP 2D6,14,15,25,38,40-42,45,46,48,67,109,161,171,192,195,205,262 the tertiary amine TCAs are demethylated by several CYP enzymes including CYP 1A2, 3A3/4 and possibly 2C19. 19,38,41,42,46,58,118,161,168,192,202,247,256,257,262 Concomitant administration of fluvoxamine inhibits such demethylation and produces higher than usual concentrations of the parent drug (ie, tertiary amine TCAs such as clomipramine) relative to their demethylated metabolite (ie, a secondary amine TCA such as desmethylclomipramine) (see Section 3).18,27,28,118 Fluvoxamine can also produce clinically significant elevations of theophylline and clozapine, drugs dependent on CYP 1A2 for their metabolism.137,260,270,274
| TABLE 8.9 — In Vivo Studies of Effects of Different SSRIs on CYP 2D6 Function | ||||||
| # | Study | Reference | Drug | Dose | Substrate | Result |
| 1 | Gram et al, 1993 | 110 | Citalopram | 40 mg/d | IMI/DMI | |
| 2 | Bergstrom et al, 1992 | 24 | Fluoxetine | 60 mg/d (7 days) | DMI | |
| 3 | Preskorn et al, 1994 | 219 | Fluoxetine | 20 mg/d (3 wks) | DMI | |
| 4 | Spina et al, 1993 | 263 | Fluovoxamine | 100 mg/d | IMI/DMI | |
| 5 | Albers et al, 1995 | 4 | Paroxetine | 30 mg/d | IMI/DMI | |
| 6 | Alderman et al, 1996 | 5 | Paroxetine | 20 mg/d | DMI | |
| 7 | Brosen et al, 1993 | 41 | Paroxetine | 20 mg/d | DMI | |
| 8 | Jann et al, 1996 | 136 | Sertraline | 50 mg/d | IMI | 0% |
| 9 | Preskorn et al, 1994 | 219 | Sertraline | 50 mg/d | DMI | |
| 10 | Alderman et al, 1996 | 5 | Sertraline | 50 mg/d | DMI | |
| 11 | Sproule et al, 1995 | 264 | Sertraline | 108 mg/d | Dextromethorpan | |
| 12 | Zussman et al, 1994 | 298 | Sertraline | 150 mg/d | DMI | |
| 13 | Kurtz et al, 1994 | 153 | Sertraline | 150 mg/d | DMI | |
| TABLE 8.10 — Relative Potency of the Enantiomers of Fluoxetine and Norfluoxetine for Inhibiting the CYP enzyme 2D6 | |
| Enantiomer | Kinetic inhibitory constant Ki (µm)1 |
| S-fluoxetine | 0.22 |
| R-fluoxetine | 1.38 |
| S-norfluoxetine | 0.31 |
| R-norfluoxetine | 1.48 |
|
S/R ratio for fluoxetine and norfluoxetine = 2.22 |
|
|
S/R ratio = ratio of plasma levels of the 2 enantiomer under steady-state conditions when the racemic mixture is being taken. |
|
Based on the results from the Brosen et al and Rasmussen et al in vitro studies, the other SSRIs would not be expected to inhibit CYP 1A2 to any meaningful extent under clinically relevant conditions (Table 8.7). While there are no formal in vivo studies, there is indirect in vivo evidence to support this conclusion. Although concomitant administration of fluvoxamine produces a substantial increase in warfarin plasma levels (+ 65%),22 this effect does not occur with fluoxetine, paroxetine or sertraline.16,245,287
Warfarin is a racemic mixture. S-warfarin is metabolized by CYP 2C9/10 and is the active enantiomer in terms of anticoagulant effect.149,162,238 The inactive R-warfarin enantiomer is metabolized by CYP 1A2 but can inhibit CYP 2C9/10 and thus produce an accumulation of the active S-warfarin enantiomer. The fact that fluvoxamine produces a buildup of warfarin plasma levels is compatible with the inhibition of CYP 1A2 and a resultant buildup of R-warfarin plasma levels which in turn inhibit CYP 2C9/10, resulting in a buildup of S-warfarin plasma levels and hence, an increase in anticoagulant effect. The fact that this scenario does not occur with sertraline, fluoxetine and paroxetine is compatible with an absence of effect of these 3 SSRIs on both CYP 1A2 and 2C9/10.
There have been no in vitro studies of the different effects of SSRIs on CYP 2C9/10. However, in vivo studies have been done with both tolbutamide and warfarin, which are substrates for this enzyme (Table 8.11). Fluoxetine and sertraline did not produce a clinically significant decrease in the clearance of tolbutamide or warfarin, and paroxetine did not affect warfarin.16,159,245,273,287 Despite these results with fluoxetine, the U.S. Food and Drug Administration has compiled 163 cases in which the concomitant use of fluoxetine elevated phenytoin plasma levels. In 23 cases with adequate data, the average increase was 161%, occurring on average 2 weeks after fluoxetine was started, and was associated with development of clinical manifestations of toxicity including ataxia, somnolence and nystagmus.71,134,249,293 These cases have led to a revision of the product labelling for fluoxetine to advise about this interaction. Since phenytoin is metabolized by CYP 2C9/10, this interaction suggests that fluoxetine inhibits this CYP enzyme. The reason for the apparent discrepancy between this effect of fluoxetine on phenytoin clearance and the absence of an effect of fluoxetine on the clearance of tolbutamide and warfarin is not known.
No in vivo studies have been done with SSRIs using an ideal model substrate for this CYP enzyme. However, studies with 3 SSRIs (ie, fluoxetine, fluvoxamine and sertraline) have been performed with diazepam which is principally dependent on CYP 2C19 for its metabolisms at least at low concentrations (Table 8.11). Demethylation of diazepam to desmethyldiazepam is the major route of elimination and is dependent on CYP 2C19 at the concentrations achieved on conventional low doses.9,26,56,132,286 At higher concentrations, this demethylation can also be mediated by CYP 3A3/4.10,294 Hydroxylation of diazepam to temazepam is typically a minor pathway and is mediated by CYP 3A3/4.26,119
Desmethyldiazepam undergoes C3-hydroxylation which may be mediated by a single, rate-limiting enzyme, CYP 2C19.26,119 In contrast, C3-hydroxylation of diazepam is minimal at low drug concentrations, increases sigmoidally with increasing concentrations, and may be mediated entirely by CYP 3A3/4.119
Diazepam thus does not fulfill the criteria of a model substrate due to its multiple pathways mediated by more than one CYP enzyme. Nonetheless, the in vivo studies that have explored the potential interaction between these three SSRIs and diazepam does provide useful information on whether they inhibit either CYP 2C19 or 3A3/4. Since the metabolism of diazepam at low substrate concentrations appears to be principally mediated by CYP 2C19, and since the in vivo studies that follow were done with low doses of diazepam and hence, at low substrate concentrations, their findings suggest the delay in diazepam clearance produced by some SSRIs is due to an effect on CYP 2C19.
Fluvoxamine (average dose = 112 mg/day) produced on average a 280% and 41% increase in the area under curve (AUC) for diazepam and desmethyldiazepam respectively. The effect on diazepam represented a 65% reduction in its apparent clearance from 0.40 to 0.14 ml/min/kg and a marked prolongation in diazepam half-life from 51 to 118 hours (p < 0.01).203 Fluoxetine, using a loading dose strategy (60 mg/d × 8 days) that would produce combined levels of fluoxetine and norfluoxetine approximately 20% to 30% lower than those that will occur at steady-state on 20 mg/day, produced a 40% increase in the AUC of diazepam.160 Another study using only 30 mg/d × 7 days did not produce an increase in diazepam AUC.159 This finding is consistent with the concentration-dependent nature of the effect since this loading dose strategy (30 mg/d × 7 days) would produce less than half the levels that would be expected under steady-state conditions on 20 mg/day. In contrast to fluvoxamine, fluoxetine (60 mg/d × 8 days) reduced the levels of metabolically derived desmethyldiazepam; which raises the possibility of a different MOA (ie, an effect on a different CYP enzyme such a CYP 3A3/4 rather than CYP 2C19). Further work is needed with fluoxetine and a more ideal substrate for CYP 2C19 to determine to what extent its effects on diazepam are due to an effect on CYP 2C19 versus CYP 3A3/4. Based on the study by Gardner and colleagues, sertraline under steady-state conditions on its usually effective, minimum dose of 50 mg/day will be expected to produce an appreciably smaller effect (ie, approximately 8% increase in AUC) than either fluvoxamine or fluoxetine.99 Thus, these studies with these 3 SSRIs suggests that CYP 2C19 is inhibited potently by fluvoxamine, possibly weakly by fluoxetine, and trivially inhibited by sertraline at each drugs' usually effective, minimum, antidepressant dose. No comment can be made about the potential impact of citalopram or paroxetine on CYP 2C19 since appropriate studies have not been done.
A large number of drugs from different therapeutic classes are dependent on 2D6 for their metabolism including TCAs, codeine, several neuroleptics, B-blockers, and Type IC antiarrhythmics (Table 7.9). Of all the CYP enzymes, 2D6 has been studied most thoroughly with respect to the different effects of SSRIs on its functional activity (Table 8.7, Table 8.9 and Figure 8.2). Based on most in vitro studies (Table 8.7), paroxetine is 2- to 4-times more potent than fluoxetine, and 5 to 20 times more potent than sertraline. Fluvoxamine and citalopram are also substantially less potent than paroxetine and fluoxetine with respect to the in vitro inhibition of 2D6.
Recall that fluoxetine is marketed as the racemate (Section 2). The in vitro data shown in Table 8.7 for the inhibition of CYP 2D6 is for the racemate. The potency of the two enantiomers of fluoxetine and norfluoxetine is shown in Table 8.10. The relative potency of these enantiomers for other CYP enzymes has not been established.
All of SSRIs, with the apparent exception of fluvoxamine, have "active" metabolites relative to the inhibition of this enzyme in vitro with a potency similar to that of the parent compound (Table 8.7). However, only norfluoxetine reaches sufficient concentrations at each drug's usually effective, minimum concentration under routine clinical conditions to contribute in a clinically meaningful way to the inhibition of this enzyme. The contribution of norfluoxetine takes on special significance due to its extended half-life (ie, 1 to 2 weeks), which means that the risk of a drug-drug interaction mediated by this active metabolite persists for weeks after fluoxetine has been discontinued.
| FIGURE 8.2 — Differential In Vivo Effects of Five Different SSRIs on CYP 2D6 Function |
 |
| References: 1110, 224, 3219, 4263, 54, 65, 741, 8136, 9264, 10298, 11153 |
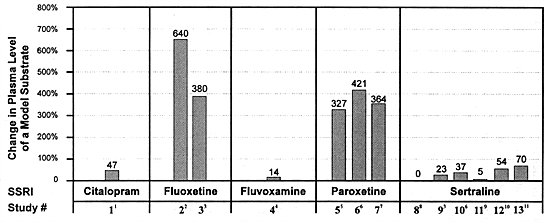
Desipramine has generally served as the model substrate for the in vivo studies of SSRIs on CYP 2D6 function (Table 8.9 and Figure 8.2). In these studies, the effect of each SSRI on the clearance of desipramine has been studied primarily at their usually effective dose: fluoxetine and paroxetine (20 mg/day) and sertraline (50 mg/day) (see Section 5). Since a fixed-dose study has not been published for citalopram or for fluvoxamine, their usually effective, minimum doses have not been as rigorously established (see Section 5). Nevertheless, they have been tested for effects on CYP 2D6 at doses of 40 mg/day for citalopram and 100 mg/day for fluvoxamine.
The results are quite clear about the relative impact of each SSRI on the in vivo clearance of desipramine: fluoxetine produced a 380% to 640% increase in desipramine levels; paroxetine, a 327% to 421% increase; citalopram, a 47% increase; sertraline, a 0% to 37% increase; and fluvoxamine, a 14% increase (Table 8.9, Figure 8.2). Thus, only 2 out of the 5 SSRIs marketed worldwide produce meaningful inhibition of this enzyme at their usually effective dose.
Only in the broadest sense can one state that all the SSRIs inhibit CYP 2D6. It is misleading to make such a claim without acknowledging the substantial differences among these drugs.
To further put the differences among the SSRIs in perspective, the above results for fluoxetine somewhat underestimate its full effect at steady-state at 20 mg/day. That is because steady-state levels of fluoxetine and norfluoxetine that would be expected at 20 mg/day were not achieved in either study due to the extended half-life of norfluoxetine. That is true even for the Bergstrom et al study which used a loading dose strategy of 60 mg/day for 7 days.
Only paroxetine and sertraline have been formally studied at doses above their usually effective dose. The study by Albers et al used a dose of 30 mg/day; however, that dose was administered for only 4 days prior to the administration of the test dose of imipramine.4 Consistent with that short treatment phase, the plasma levels of paroxetine in this study (10-30 mg/ml) were similar to the 2 studies which gave 20 mg per day for at least 10 days.5,41 Thus, the study by Albers et al was more of replication of those 2 earlier studies than a test of a higher dose.
Sertraline has been the one SSRI most extensively tested at doses above its usually effective antidepressant dose. Sproule et al found a 5% prolongation in the clearance of dextromethophan following treatment with sertraline 100 mg/day for 21 days.264 Two separate studies examined the effect of sertraline 150 mg/day administered for a sufficient duration to achieve steady-state and found a 54% to 70% increase in desipramine plasma levels.153,298 Thus sertraline at its usually effective dose of 50 mg/day produces a 0% to 37% increase in the plasma levels of a model substrate such as desipramine which is principally dependent on CYP 2D6 for its clearance, a 5% prolongation in clearance at 108 mg/day, and a 54% to 70% increase in plasma levels at 150 mg/day. The magnitude of this effect is substantially less at all of these doses than the effect of fluoxetine and paroxetine at their usually effective minimum dose.
While citalopram and fluvoxamine have been less extensively studied than sertraline, their effects on CYP 2D6 are also substantially less than fluoxetine and paroxetine (Table 8.9, Figure 8.2).
|
|||||
|
Figures 8.3 and 8.4 demonstrate the concentration-dependent nature of the effects of fluoxetine, paroxetine and sertraline on CYP 2D6 as reflected in the changes in plasma levels of the CYP 2D6 substrate, desipramine. These figures are from the formal in vivo pharmacokinetic studies that compared the effects of fluoxetine 20 mg/day to sertraline 50 mg/day in one study219 and the effects of paroxetine 20 mg/day to sertraline 50 mg/day in a second study.5 On the X-axis of the graphs is the concentration of each respective SSRI and on the Y-axis is the plasma concentration of desipramine. There is a robust relationship between increasing plasma levels of fluoxetine plus norfluoxetine and increasing levels of desipramine in Figure 8.3. The same is true for paroxetine and desipramine plasma levels in Figure 8.4. In both figures, the relationship between increasing plasma levels of sertraline and elevations of desipramine plasma levels is considerably more modest. These results are fully consistent with the differences in the in vitro potency of these 3 SSRIs for the inhibition of CYP 2D6 and the differences in concentrations of 3 SSRIs at this enzyme at each drug's usually effective, minimum dose. Table 10.1 provides further illustration of the concentration-dependent nature of the inhibition of CYP enzymes.
To put these results in perspective, fluoxetine and paroxetine appear to produce approximately 85% inhibition of CYP 2D6 activity versus 15% inhibition or less with citalopram, fluvoxamine or sertraline (Table 8.4).
With the exception of citalopram, all of the SSRIs and their primary metabolites have been studied in terms of their in vitro effects on the metabolism of alprazolam, which is a substrate for the CYP 3A3/4 enzyme (Table 8.7). Fluvoxamine and norfluoxetine were the most potent in vitro inhibitors of the SSRIs. Sertraline and paroxetine were intermediate. Fluoxetine, the parent drug, was the weakest. To keep these results in perspective, the in vitro potency of both fluvoxamine and norfluoxetine are considerably less potent than the antifungal agent, ketoconazole.278
This antifungal agent can produce clinically serious elevations of terfenadine due to its inhibition of CYP 3A3/4.29,126,127,279,297 Based on the results of in vitro studies, coadministration of fluvoxamine and fluoxetine (due to norfluoxetine) would be predicted to cause more in vivo inhibition of CYP 3A3/4-dependent drug metabolism than would either paroxetine or sertraline due to the difference in plasma concentrations of these different agents at comparable antidepressant doses of each agent (Table 8.4 and Section 10, Appendix) but substantially less than ketoconazole.
Unfortunately, this prediction has only been partially tested by formal in vivo studies. The in vivo effects of fluoxetine and fluvoxamine on the clearance of alprazolam, a model substrate for CYP 3A3/4, has been tested in vivo, but similar studies have not been done with citalopram, paroxetine or sertraline (Table 8.11). Fluvoxamine (100 mg/day for 10 days) produced a doubling of alprazolam plasma levels with a 55% decrease in its clearance.87 This finding is compatible with the in vitro data and the known plasma levels of fluvoxamine that would be expected at this dose.
In the first study with fluoxetine, a 33% increase in alprazolam plasma levels occurred after only 4 days of coadministration of fluoxetine 60 mg/day (ie, a loading dose strategy).157 In the second study, the loading dose strategy for fluoxetine was 40 mg/day for 10 days which produced a combined plasma level of fluoxetine and norfluoxetine of 160 ng/ml, which is approximately 25% less than the steady-state levels of 200 ng/ml that should occur on 20 mg/day.112 In this study, fluoxetine produced a 25% decrease in the clearance of alprazolam. Based on the results of these 2 studies, fluoxetine, at steady-state on 20 mg/day, will be expected to produce a 30% to 40% increase in alprazolam plasma levels. As with the inhibition of CYP 2D6, the effect of norfluoxetine on CYP 3A3/4 can last for an extended interval after fluoxetine discontinuation. In the second study, the increase in alprazolam plasma levels persisted for more than 2 weeks after norfluoxetine was discontinued.112
There have been some other studies relative to CYP 3A3/4 substrates which deserve some comment (Table 8.11). Carbamazepine is in part metabolized by CYP 3A3/4.143,172,207 However, carbamazepine is not a model substrate due to the fact that it induces its own metabolism as well as the metabolism of other drugs. Thus, interaction studies with carbamazepine are not easily interpreted. With this caveat in mind, it is noteworthy that fluoxetine-induced slowing of carbamazepine metabolism has been reported by some but not all investigators.101,102,113,201,261 There is also a case report describing cardiac abnormalities occurring 30 days after fluoxetine was added to a regimen containing terfenadine and resolved when terfenadine was stopped.268 This case, coupled with the in vitro and in vivo data discussed above, suggests the need for a formal study given the widespread use of fluoxetine and terfenadine, coupled with the potential seriousness of a significant interaction. The available data suggest that fluoxetine at 20 mg/day is unlikely to produce sufficient inhibition of CYP 3A3/4 to produce a clinically significant interaction with terfenadine.
| TABLE 8.11 — Comparison of the In Vivo Effects of Different SSRIs on Specific CYP Enzyme substrates* | |||||
| SSRI | Changes in Plasma Levels | ||||
| CYP 2C9/10 | CYP 2C19 | CYP 3A3/4 | |||
| TBA | PHT | DZ | APZ | CBZ | |
| Fluoxetine | 50%3 | 27%6 0-63%7 |
|||
| Fluvoxamine | NA | NA | 30-70%10 | ||
| Paroxetine | 0%11 | NA | NA | NA | 0%12 |
| Sertraline | 0%14 | NA | 0%16 | ||
|
* CYP 2C9/10: TBA = tolbutamide, PHT
= phenytoin |
|||||
|
|||||
There are also case reports of 30% to 70% increases in carbamazepine levels when 100 to 300 mg/day of fluvoxamine is coadministered.35,96 In a formal study, sertraline at a dose of 200 mg/day (which is four-times its usually effective, minimum dose) did not alter carbamazepine levels.74 In a controlled-case series as opposed to a formal pharmacokinetic study, paroxetine did not alter carbamazepine levels.8
Taken as a whole, these studies indicate that CYP 3A3/4 inhibition produced by SSRIs at their usually effective dose ranges from moderate to not detectable as follows: fluvoxamine (moderate) > fluoxetine (mild) > paroxetine and sertraline (not detectable) (Table 8.7). There are a few caveats to this statement. First, citalopram is not mentioned because it has not been studied adequately. Second, the effects on these enzymes is concentration dependent so that higher doses could produce greater effect. This is particularly important for SSRIs, which have nonlinear pharmacokinetics (see Section 7). For example, fluoxetine produces mild inhibition of CYP 3A3/4 at 20 mg/day, but will be expected to increase disproportionately with dose increases. The mild inhibition (20% to 50% change) will not be expected to produce a clinically meaningful drug-drug interaction in most instances, but the likelihood and severity of a clinically meaningful interaction increases as the magnitude of the change in clearance due to the degree of enzyme inhibition increases (see Appendix).
To put these results in perspective, it is important to recognize the substantial difference between the inhibition produced by fluvoxamine and fluoxetine and the effects of drugs such as ketoconazole. That has unfortunately not been done in several recent review articles.301,302 These papers have listed several SSRIs as inhibitors of CYP 3A3/4 along with drugs such as ketoconazole without acknowledging the difference in the magnitude of their effects. The profound inhibition of CYP 3A3/4 enzyme produced by ketoconazole and itraconazole is responsible for the potentially fatal interaction of these drugs with terfenadine. While fluvoxamine is the most potent SSRI in vitro with regard to CYP 3A3/4 inhibition, it is three orders of magnitude less potent than ketoconazole. In vivo studies have confirmed that there is a 20- to 100-fold difference between the effects of fluvoxamine and fluoxetine on the CYP 3A3/4 mediated biotransformation of model substrates such as triazolobenzodiazepines and the effects of antifungal agents such as ketoconazole and itraconazole under clinically relevant dosing conditions (Figure 8.5). Failure to recognize these substantial differences can cause significant confusion about the relative risk of experiencing a serious adverse drug-drug interaction due to CYP 3A3/4 inhibition.
| FIGURE 8.5 — Relative In Vivo Effects of CYP 3A3/4 Inhibitors on Triazolobenzodiazepines (TBZ) |
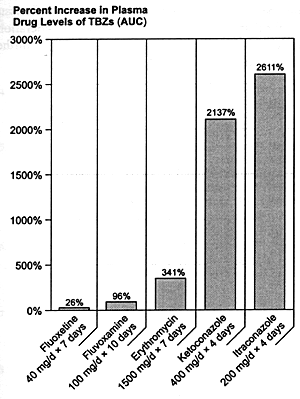 |
| AUC = Area under curve. |
| References: 303-306 |
Table 8.11 summarizes the in vivo data which is available for the 4 most extensively studied SSRIs in terms of their effects on CYP enzymes other than CYP 2D6. The results for CYP 2D6 are summarized in Table 8.9 and Figure 8.2. Since the inhibition of these enzymes is concentration-dependent, it is important to ensure an adequate trial of the usually effective, minimum dose, particularly for SSRIs such as fluvoxamine and fluoxetine which inhibit more than one CYP enzyme. The data are clear that the effect of the SSRIs on specific CYP enzymes is an SSRI-specific issue rather than a class issue and involves considerations beyond the in vitro inhibition constant (Ki) as further discussed in Section 10, Appendix.